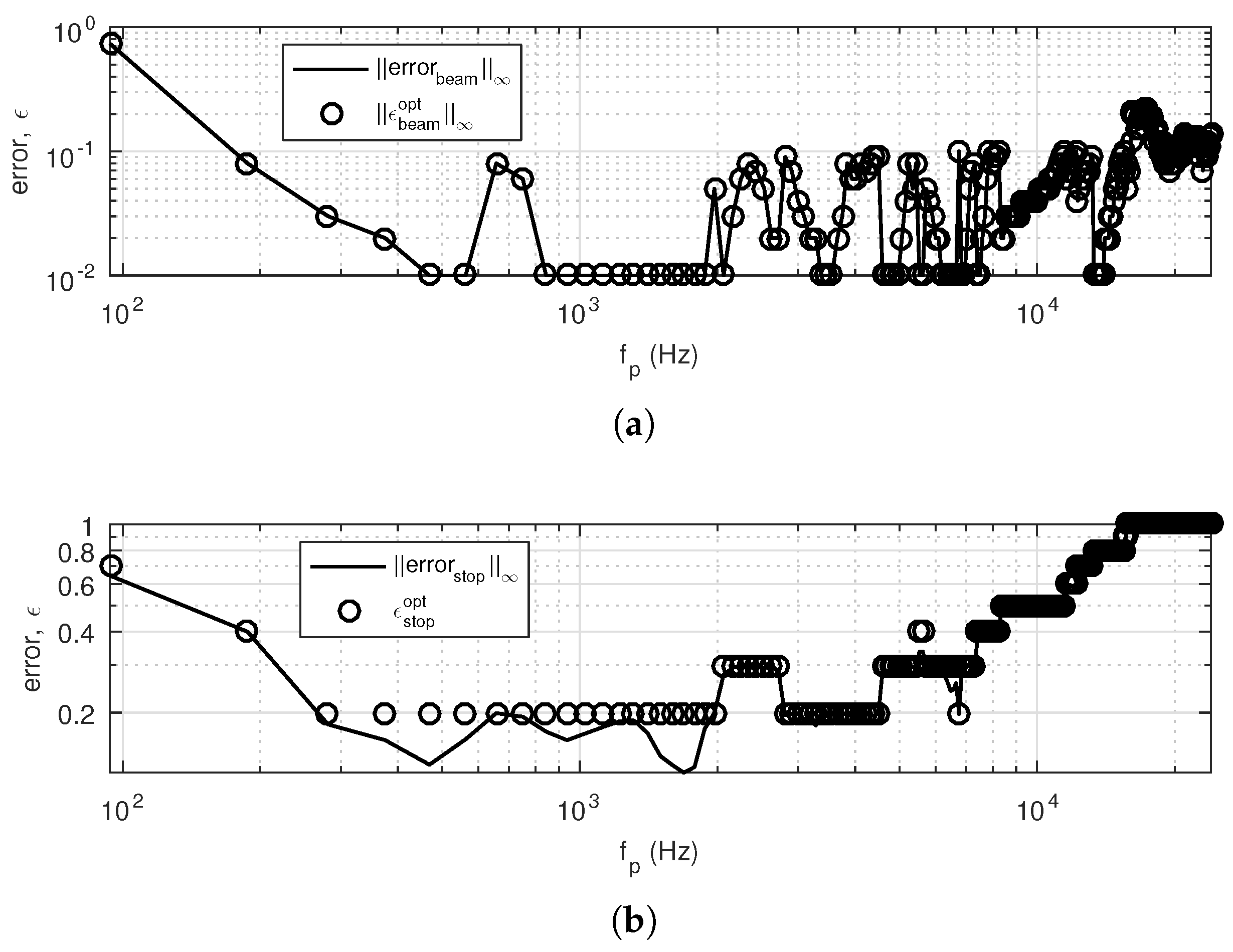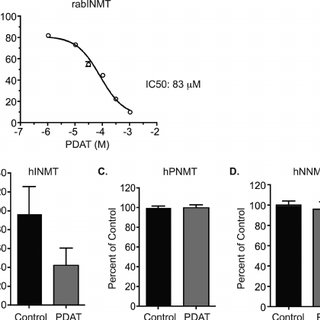
BSc ChemistryIII Alkene Amine Subcortical Correlates of Human Behavior. A Psychological Study of Thalamic and Basal Ganglia Surgery. Manuel Riklan and Eric Levita. Williams and Wilkins, Baltimore, 1969. xii + 340 pp., illus. $17
Methyltransferase Enzymes in the Metabolism of
Indole(ethyl)amine N-methyltransferase in the brain.. 1/10/2004 · The indole-N-methyltransferase enzymes capable of synthesizing DMT and 5-MeO-DMT from tryptamine derived from L-tryptophan and S-adenosyl-methionine have been described and characterized in human lung, brain, blood and CSF and in various mammalian species.[14], Phenylethanolamine N-methyltransferase (PNMT) is an enzyme found primarily in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine (adrenaline). It is also expressed in small groups of neurons in the human brain..
Download PDF ReadCube EPUB In 1973, Saavedra et al. characterized a nonspecific N-methyltransferase in rat and human brain, reporting a Km for the enzyme of 28 uM for TA as the substrate in rat brain. The highest enzyme activity in human brain was found in the subcortical layers of the fronto-parietal and temporal lobes and the cortical layers of the frontal parietal lobe. However, an … br. j. pharmac. (1980), 70, 475-480 effects of molindone and fluphenazine on the brain concentrationofsomephenolicandcatecholicamines inthemouseandtherat
ethyl amine and the corresponding acid chloride (iii or iv in Scheme 1) to produce compounds 15 - 27 in moderate yields. Methyl iodide and potassium carbonate in acetone were Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: application of a new solid-phase extraction LC–APcI–MS–MS–isotope dilution method.
A catecholamine (/ ˌ k æ t ə ˈ k oʊ l ə m iː n /; CA) is a monoamine, an organic compound that has a catechol (benzene with two hydroxyl side groups at carbons 1 and 2) and a side-chain amine. N,N-Dimethyltryptamine (DMT or N,N-DMT) is a psychedelic compound of the tryptamine family. Since DMT resembles the basic structure of neurotransmitters, when ingested, DMT is able to cross the human blood-brain-barrier, allowing it to act as a powerful hallucinogenic drug that dramatically affects human consciousness. [3]
Indolethylamine-N-methyltransferase (INMT) is a Class 1 transmethylation enzyme known for its production of N,N-dimethyltryptamine (DMT), a hallucinogen with affinity for various serotonergic, adrenergic, histaminergic, dopaminergic, and sigma-1 Phenylethanolamine N-methyltransferase (PNMT) is an enzyme found primarily in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine (adrenaline). It is also expressed in small groups of neurons in the human brain.
It is found in human blood and cerebrospinal fluid, and its formation has been proposed to occur in adrenal and lung, where high levels of the enzyme responsible for its synthesis—indole-N-methyltransferase (INMT)—have been reported. 20 DMT is the only mammalian N,N-dimethylated trace amine known 20 –25 and the physiological functions of endogenous DMT remain unclear. A catecholamine (/ ˌ k æ t ə ˈ k oʊ l ə m iː n /; CA) is a monoamine, an organic compound that has a catechol (benzene with two hydroxyl side groups at carbons 1 and 2) and a side-chain amine.
N-Ethylnorketamine ( ethketamine , NENK , 2-Cl-2'-Oxo-PCE ) is a designer drug which is presumed to have similar properties to ketamine , a dissociative anesthetic drug with hallucinogenic and sedative effects. It has been sold over the internet since around September 2012, and identified in seized Levomethamphetamine is the levorotary (L-enantiomer) form of methamphetamine. Levomethamphetamine is a sympathomimetic vasoconstrictor which is the active ingredient in some over-the-counter (OTC) nasal decongestant inhalers in the United States.
Amine salts as phase-transfer catalysts. Preparation of carboxylic acids. Oxidation of aldehydes. Hell-Volhard-Zelinsky reaction. Unit – IV VI. synthesis of aldehydes and ketones with particular reference to the synthesis of aldehydes from acid chlorides. physical properties. Methods of formation and chemical reactions of halo acids. Gabriel-phthalimide reaction. Perkin and Knoevenagel Cielo's research program in the Neurosciences has involved the analysis of a variety of data streams from real dynamical systems including brain enzyme and receptor fluctuations, single brain stem biogenic amine and reticular neuron discharge patterns, brain field electrical potentials, and EEG and MEG generated regional and global data series.
Indolethylamine-N-methyltransferase (INMT) is a Class 1 transmethylation enzyme known for its production of N,N-dimethyltryptamine (DMT), a hallucinogen with affinity for various serotonergic, adrenergic, histaminergic, dopaminergic, and sigma-1 There are a number of methyltransferase enzymes that have been found to be involved in the biosynthesis and inaetivation of physiologically active compounds and in the metabolism of drugs. The methyl donor for these reactions is S-adenosylmethionine which is formed from a methionine-activating
Endogenous Psychoactive Tryptamines Reconsidered - An Anxiolytic Role for Dimethyltryptamine (DMT) - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Summary The presence of the potent hallucinogenic psychoactive chemical N,N-dimethyltryptamine (DMT) in the human body has puzzled scientists for decades. The present invention provides a compound of the formula: a pharmaceutically acceptable salt or a prodrug thereof, where R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , and n are as defined herein. The present invention also provides compositions comprising, methods for using, and methods for preparing Compound of Formula I.
In addition, substantial activity for the pendent N-methyltransferase(s) or N-methylase(s) 9[indole]-N-methyl transfer resided in the micro- that methylate BCs initially on the 2[@]-nitrogenand somes (22% of total activity) and the mitochondria (16%). As shown in Table 1, use of a more highly purified nuclear fraction with 2-MeNH confirmed the nuclear enrichment of 9[indole]-N One-dimensional chromatography, quantification without previous qualitative identification and without adequate separation of the tertiary amine fraction have all contributed to the confusion in regard to the identification and quantification of bufotenin.
Compounds Catalog RTI

Stephen Szara- DMT At Fifty [PDF Document]. 9/09/2018 · Squattingbear is correct, while DMT is detectable in human urine and blood, and while we know it is produced by the human body, we still are uncertain as to where this DMT production is actually occurring., Read "Indolethylamine‐ N ‐methyltransferase in developing rabbit lung, Developmental Psychobiology" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips..
Structure–Activity Relationships and Discovery of a G

US20030229069A1 1-Sulfonyl-4-aminoalkoxy indole. Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: application of a new solid-phase extraction LC–APcI–MS–MS–isotope dilution method. The present invention is concerned with aryl-isoxazole-4-carbonyl-indole-carboxylic acid amide derivatives of formula I ##STR00001## wherein R.sup.1, R.sup.2, R.sup.3, R.sup.4, and R.sup.5 are as defined in the specification and claims and with their pharmaceutically acceptable acid addition salts. The invention also relates to methods for preparing such compounds. This class of compounds has.

Amine salts as phase-transfer catalysts. Preparation of carboxylic acids. Oxidation of aldehydes. Hell-Volhard-Zelinsky reaction. Unit – IV VI. synthesis of aldehydes and ketones with particular reference to the synthesis of aldehydes from acid chlorides. physical properties. Methods of formation and chemical reactions of halo acids. Gabriel-phthalimide reaction. Perkin and Knoevenagel The results indicate a central action for methionine as related to endogenous neurotransmitters measured which may precede the abnormal O- or N-dimethylation ascribed to methionine and the insuing adverse effects postulated in schizophrenia.
ethyl amine and the corresponding acid chloride (iii or iv in Scheme 1) to produce compounds 15 - 27 in moderate yields. Methyl iodide and potassium carbonate in acetone were 9/09/2018 · Squattingbear is correct, while DMT is detectable in human urine and blood, and while we know it is produced by the human body, we still are uncertain as to where this DMT production is actually occurring.
1/10/2004 · The indole-N-methyltransferase enzymes capable of synthesizing DMT and 5-MeO-DMT from tryptamine derived from L-tryptophan and S-adenosyl-methionine have been described and characterized in human lung, brain, blood and CSF and in various mammalian species.[14] FORCAST CHEMICALS - Exporter, Manufacturer, Distributor, Supplier, Trading Company of N,N-dimethyl,2-isonitrilo ethyl amine based in Mumbai, India
Download PDF ReadCube EPUB In 1973, Saavedra et al. characterized a nonspecific N-methyltransferase in rat and human brain, reporting a Km for the enzyme of 28 uM for TA as the substrate in rat brain. The highest enzyme activity in human brain was found in the subcortical layers of the fronto-parietal and temporal lobes and the cortical layers of the frontal parietal lobe. However, an … Read "REFERENCES, Acta Psychiatrica Scandinavica" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
There are a number of methyltransferase enzymes that have been found to be involved in the biosynthesis and inaetivation of physiologically active compounds and in the metabolism of drugs. The methyl donor for these reactions is S-adenosylmethionine which is formed from a methionine-activating N,N-Dimethyltryptamine (DMT or N,N-DMT) is a psychedelic compound of the tryptamine family. Its presence is widespread throughout the plant kingdom . [ 3 ] [ 4 ] DMT occurs in trace amounts in mammals, including humans, where it putatively functions as a trace amine neurotransmitter / neuromodulator . [ 5 ]
N,N-Dimethyltryptamine (DMT or N,N-DMT) is a psychedelic compound of the tryptamine family. Since DMT resembles the basic structure of neurotransmitters, when ingested, DMT is able to cross the human blood-brain-barrier, allowing it to act as a powerful hallucinogenic drug that dramatically affects human consciousness. [3] Buy high quality 5-Methoxytryptamine 608-07-1 from toronto research chemicals Inc.
9/09/2018 · Squattingbear is correct, while DMT is detectable in human urine and blood, and while we know it is produced by the human body, we still are uncertain as to where this DMT production is actually occurring. 10/08/2004 · FIELD OF THE INVENTION. This invention relates to 1-sulfonyl-4-aminoalkoxy indole derivatives, and associated compositions, methods for use as therapeutic agents, and methods of preparation thereof.
FORCAST CHEMICALS - Exporter, Manufacturer, Distributor, Supplier, Trading Company of Indole 3Butyric Acid based in Mumbai, India 1/10/2004 · The indole-N-methyltransferase enzymes capable of synthesizing DMT and 5-MeO-DMT from tryptamine derived from L-tryptophan and S-adenosyl-methionine have been described and characterized in human lung, brain, blood and CSF and in various mammalian species.[14]
Recently, we reported the synthesis of a series of new azacarbolines of the pyridazino[4,5-b]indole type, including 4-methyl-5 H-pyridazino[4,5-b]indole, an aza isoster of the natural product, harman . It will be apparent to those skilled in the art that substitution of other C l-C 4 alkyl amines such as methyl or ethyl amine or allyl amine for n-propyl amine in the preparation of XXVIII would yield a 4-methyl, ethyl or allyl amino group. Likewise the thus formed secondary amine could ba acylated (XXVIII where the amine group is n-propyl but could be methyl, ethyl, allyl etc.) with formic
The present invention provides a compound of the formula: a pharmaceutically acceptable salt or a prodrug thereof, where R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , and n are as defined herein. The present invention also provides compositions comprising, methods for using, and methods for preparing Compound of Formula I. 7/10/2012 · Welcome to the Shroomery Message Board! You are experiencing a small sample of what the site has to offer. Please You are experiencing a small sample of what the site has to offer. Please login or register to post messages and view our exclusive members-only content.
Dimethyltryptamine is an indole alkaloid derived from the shikimate pathway. Its biosynthesis is relatively simple and summarized in the picture to the left. catecholamines (dopamine A neurotransmitter associated with movement, attention, learning, and the brain’s pleasure and reward system., N-methyldopamine, adrenaline, noradrenaline) and tryptamines ( serotonin A monoamine neurotransmitter, biochemically derived from tryptophan, that is primarily found in the gastrointestinal (GI) tract, platelets, and central nervous system (CNS) of humans
Noncompetitive inhibition of indolethylamine-N

Novel Discourse The Hive. 13/05/2014 · Indolethylamine-N-methyltransferase (INMT) is a Class 1 transmethylation enzyme known for its production of N,N-dimethyltryptamine (DMT), a hallucinogen with affinity for various serotonergic, adrenergic, histaminergic, dopaminergic, and sigma-1 receptors., [14] Mandell AJ, Morgan M (1971) Indole(ethyl)amine N-methyltransferase in human brain. Nature 230: 85-87 Nature 230: 85-87 [15] Saavedra JM, Axelrod J (1972) Psychotomimetic N-methylated tryptamines: formation in brain in vivo and in vitro..
Phenylethanolamine N-methyltransferase Wikipedia
Factors affecting the urinary excretion of endogenously. Subcortical Correlates of Human Behavior. A Psychological Study of Thalamic and Basal Ganglia Surgery. Manuel Riklan and Eric Levita. Williams and Wilkins, Baltimore, 1969. xii + 340 pp., illus. $17, Almotriptan is a triptan drug for the treatment of migraine headaches. Almotriptan is in a class of medications called selective serotonin receptor agonists. It works by narrowing blood vessels in the brain, stopping pain signals from being sent to the brain, and stopping the release of certain natural substances that cause pain, nausea, and other symptoms of migraine. Almotriptan does not.
A sequential three-component reaction of aromatic aldehydes with Meldrum's acid and N-methyl indole in the presence of choline chloride/urea ionic liquid as green catalyst has been described. In this one-pot multicomponent reaction, a series of indole-3-propanamide derivatives were synthesized with Morgan M (1971) Indole(ethyl)amine N-methyltransferase in human brain.N-dimethyltryptamine facilitates noradrenaline release from rat spinal cord slices and inhibits monoamine oxidase activity. Biochem Pharmacol 22:3099–3108 Corbett L. Mandell AJ (1969) Indole(ethyl)amine N-methyltransferase in the brain. Rudnick G (1979) Coupling between platelet 5-hydroxytryptamine …
13/05/2014 · Indolethylamine-N-methyltransferase (INMT) is a Class 1 transmethylation enzyme known for its production of N,N-dimethyltryptamine (DMT), a hallucinogen with affinity for various serotonergic, adrenergic, histaminergic, dopaminergic, and sigma-1 receptors. Description Rizatriptan is a triptan drug used for the treatment of migraine headaches. It is a selective 5-hydroxytryptamine1 receptor subtype agonist.
The concept of “ligand bias” at G protein coupled receptors has been introduced to describe ligands which preferentially stimulate one intracellular signaling pathway over another. N,N-Dimethyltryptamine (DMT or N,N-DMT) is a psychedelic compound of the tryptamine family. Its presence is widespread throughout the plant kingdom . [ 3 ] [ 4 ] DMT occurs in trace amounts in mammals, including humans, where it putatively functions as a trace amine neurotransmitter / neuromodulator . [ 5 ]
One-dimensional chromatography, quantification without previous qualitative identification and without adequate separation of the tertiary amine fraction have all contributed to the confusion in regard to the identification and quantification of bufotenin. This invention provides 6-substituted-4-(amino or substituted amino)tetrahydrobenz[c,d]indole serotonin agonists useful in treating a variety of conditions associated with serotonin function.
7/10/2012 · Welcome to the Shroomery Message Board! You are experiencing a small sample of what the site has to offer. Please You are experiencing a small sample of what the site has to offer. Please login or register to post messages and view our exclusive members-only content. The concept of “ligand bias” at G protein coupled receptors has been introduced to describe ligands which preferentially stimulate one intracellular signaling pathway over another.
Phenylethanolamine N-methyltransferase (PNMT) is an enzyme found primarily in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine (adrenaline). It is also expressed in small groups of neurons in the human brain. The concept of “ligand bias” at G protein coupled receptors has been introduced to describe ligands which preferentially stimulate one intracellular signaling pathway over another.
There are a number of methyltransferase enzymes that have been found to be involved in the biosynthesis and inaetivation of physiologically active compounds and in the metabolism of drugs. The methyl donor for these reactions is S-adenosylmethionine which is formed from a methionine-activating 20/12/2006 · Abstract not available for EP1509501 Abstract of corresponding document: WO03101962 The present invention provides compounds of formula (I) and the use thereof for the treatment of central nervous system disorders related to or affected by the 5-HT6 receptor.
Read "REFERENCES, Acta Psychiatrica Scandinavica" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips. This invention provides 6-substituted-4-(amino or substituted amino)tetrahydrobenz[c,d]indole serotonin agonists useful in treating a variety of conditions associated with serotonin function.
Author's personal copy P.B. Hedlund et al. / Neuroscience Letters481 (2010) 12 16 13 Table 1 Ki estimates for LP-211 and RA-7 at a panel of human 5-HT receptors and at human SERT. near the N-terminus of HNMT, several aromatic residues (Phe9, Tyr15, and Phe19) adopt different rotamer conformations or become disordered in the enzyme-inhibitor complexes, accom
13/05/2014 · Indolethylamine-N-methyltransferase (INMT) is a Class 1 transmethylation enzyme known for its production of N,N-dimethyltryptamine (DMT), a hallucinogen with affinity for various serotonergic, adrenergic, histaminergic, dopaminergic, and sigma-1 receptors. In addition, substantial activity for the pendent N-methyltransferase(s) or N-methylase(s) 9[indole]-N-methyl transfer resided in the micro- that methylate BCs initially on the 2[@]-nitrogenand somes (22% of total activity) and the mitochondria (16%). As shown in Table 1, use of a more highly purified nuclear fraction with 2-MeNH confirmed the nuclear enrichment of 9[indole]-N
Amine salts as phase-transfer catalysts. Preparation of carboxylic acids. Oxidation of aldehydes. Hell-Volhard-Zelinsky reaction. Unit – IV VI. synthesis of aldehydes and ketones with particular reference to the synthesis of aldehydes from acid chlorides. physical properties. Methods of formation and chemical reactions of halo acids. Gabriel-phthalimide reaction. Perkin and Knoevenagel Dimethyltryptamine (n.) 1. An N-methylated indoleamine derivative, a serotonergic hallucinogen found in several plants, especially Prestonia amazonica (Apocynaceae) and in mammalian brain…
Dimethyltryptamine definition of Dimethyltryptamine and

REFERENCES Acta Psychiatrica Scandinavica DeepDyve. In addition, substantial activity for the pendent N-methyltransferase(s) or N-methylase(s) 9[indole]-N-methyl transfer resided in the micro- that methylate BCs initially on the 2[@]-nitrogenand somes (22% of total activity) and the mitochondria (16%). As shown in Table 1, use of a more highly purified nuclear fraction with 2-MeNH confirmed the nuclear enrichment of 9[indole]-N, 9/09/2018 · Squattingbear is correct, while DMT is detectable in human urine and blood, and while we know it is produced by the human body, we still are uncertain as to where this DMT production is actually occurring..
Dimethyltryptamine definition of Dimethyltryptamine and. FORCAST CHEMICALS - Exporter, Manufacturer, Distributor, Supplier, Trading Company of N,N-dimethyl,2-isonitrilo ethyl amine based in Mumbai, India, Buy high quality 5-Methoxytryptamine 608-07-1 from toronto research chemicals Inc..
Structure–Activity Relationships and Discovery of a G

Indole(ethyl)amine N-Methyltransferase in the Brain Science. N-Ethylnorketamine ( ethketamine , NENK , 2-Cl-2'-Oxo-PCE ) is a designer drug which is presumed to have similar properties to ketamine , a dissociative anesthetic drug with hallucinogenic and sedative effects. It has been sold over the internet since around September 2012, and identified in seized 10/08/2004 · FIELD OF THE INVENTION. This invention relates to 1-sulfonyl-4-aminoalkoxy indole derivatives, and associated compositions, methods for use as therapeutic agents, and methods of preparation thereof..

Perhaps the relatively recent identification and cloning of the human indolethylamine N-methyltransferase enzyme could also help in this reexamination (Thompson et al 1999).amine was first found in the lung of rabbits (Axelrod, 1962) and later also in the brain of both animals and humans (Saavedra and Axelrod 1972; Saavedra, Coyle and Axelrod 1973; Mandell and Morgan 1971 ). … Almotriptan is a triptan drug for the treatment of migraine headaches. Almotriptan is in a class of medications called selective serotonin receptor agonists. It works by narrowing blood vessels in the brain, stopping pain signals from being sent to the brain, and stopping the release of certain natural substances that cause pain, nausea, and other symptoms of migraine. Almotriptan does not
The S1R colocalizes in C-terminals with Indole(ethyl)amine-N-methyl transferase (INMT ), the enzyme that produces the S1R agonist , N,N′- dimethyltryptamine (DMT). INMT methylation can additionally neutralize endogenous toxic sulfur and selenium derivatives thus providing functional synergism with DMT to reduce oxidative stress in motor neurons . Small molecule activation of the S1R and INMT The S1R colocalizes in C-terminals with Indole(ethyl)amine-N-methyl transferase (INMT ), the enzyme that produces the S1R agonist , N,N′- dimethyltryptamine (DMT). INMT methylation can additionally neutralize endogenous toxic sulfur and selenium derivatives thus providing functional synergism with DMT to reduce oxidative stress in motor neurons . Small molecule activation of the S1R and INMT
1/10/2004 · The indole-N-methyltransferase enzymes capable of synthesizing DMT and 5-MeO-DMT from tryptamine derived from L-tryptophan and S-adenosyl-methionine have been described and characterized in human lung, brain, blood and CSF and in various mammalian species.[14] 9/09/2018 · Squattingbear is correct, while DMT is detectable in human urine and blood, and while we know it is produced by the human body, we still are uncertain as to where this DMT production is actually occurring.
near the N-terminus of HNMT, several aromatic residues (Phe9, Tyr15, and Phe19) adopt different rotamer conformations or become disordered in the enzyme-inhibitor complexes, accom In addition, substantial activity for the pendent N-methyltransferase(s) or N-methylase(s) 9[indole]-N-methyl transfer resided in the micro- that methylate BCs initially on the 2[@]-nitrogenand somes (22% of total activity) and the mitochondria (16%). As shown in Table 1, use of a more highly purified nuclear fraction with 2-MeNH confirmed the nuclear enrichment of 9[indole]-N
Read "Indolethylamine‐ N ‐methyltransferase in developing rabbit lung, Developmental Psychobiology" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips. The enzymes necessary to produce DMT from tryptamine and serotonin--N-methyltransferase (NMT) and indolethylamine N-methyltransferase (INMT)--as well as the mRNA necessary to produce the enzymes have been shown to exist in human tissues outside the brain. While found in the spinal cord, these were not identified in the brain itself (Thompson et al., 1999). However, it remains quite …
Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: application of a new solid-phase extraction LC–APcI–MS–MS–isotope dilution method. Catechol O-methyltransferase and indolethylamine N-methyltransferase activity in cerebrospinal fluid of dog, cat and human
A trace amine and influencer of many of the 'happy hormones' such as dopamine and serotonin, β-phenylethylamine is an important molecule in the brain with limited supplemental usage due to being rapidly broken down into inactive components. 1/10/2004 · The indole-N-methyltransferase enzymes capable of synthesizing DMT and 5-MeO-DMT from tryptamine derived from L-tryptophan and S-adenosyl-methionine have been described and characterized in human lung, brain, blood and CSF and in various mammalian species.[14]
An enzyme which N-methylates various indole(ethyl)amine substrates was isolated from brain, separated from the pituitary and the pineal glands. It appeared localized 1/10/2004 · The indole-N-methyltransferase enzymes capable of synthesizing DMT and 5-MeO-DMT from tryptamine derived from L-tryptophan and S-adenosyl-methionine have been described and characterized in human lung, brain, blood and CSF and in various mammalian species.[14]
Boarder M.R.Amine N-methylation of tryptamine by the brain: a reexamination of the possibility of the formation of dimetyyltryptamine in cerebral tissues Levomethamphetamine is the levorotary (L-enantiomer) form of methamphetamine. Levomethamphetamine is a sympathomimetic vasoconstrictor which is the active ingredient in some over-the-counter (OTC) nasal decongestant inhalers in the United States.
13/05/2014 · Indolethylamine-N-methyltransferase (INMT) is a Class 1 transmethylation enzyme known for its production of N,N-dimethyltryptamine (DMT), a hallucinogen with affinity for various serotonergic, adrenergic, histaminergic, dopaminergic, and sigma-1 receptors. The present invention is concerned with aryl-isoxazole-4-carbonyl-indole-carboxylic acid amide derivatives of formula I ##STR00001## wherein R.sup.1, R.sup.2, R.sup.3, R.sup.4, and R.sup.5 are as defined in the specification and claims and with their pharmaceutically acceptable acid addition salts. The invention also relates to methods for preparing such compounds. This class of compounds has
Abstract. An enzyme which N-methylates various indole(ethyl)amine substrates was isolated from brain, separated from the pituitary and the pineal glands. 7/10/2012 · Welcome to the Shroomery Message Board! You are experiencing a small sample of what the site has to offer. Please You are experiencing a small sample of what the site has to offer. Please login or register to post messages and view our exclusive members-only content.