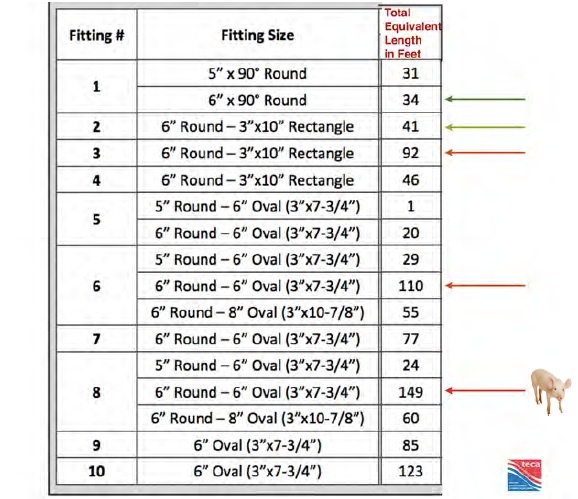
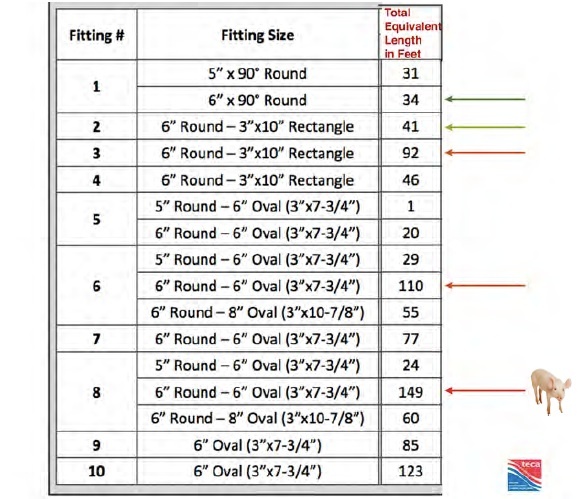
A 10-years retrospective study on Severe Cutaneous Adverse 3/10/2016 · A severe cutaneous adverse reaction, or SCAR, refers to several distinct conditions. Acute generalised exanthematous pustulosis (AGEP) Drug-induced hypersensitivity syndrome (DIHS) , also known as drug reaction with eosinophilia and systemic symptoms (DRESS)
Review of common cutaneous adverse drug reactions
Severe cutaneous adverse reactions a 4-year experience in. In the field of severe cutaneous adverse drug reactions (cutaneous ADR) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug-induced hypersensitivity syndrome (DHIS) or drug rash with eosinophilia and systemic symptoms (DRESS), major advances have recently been gained through studies of an association between HLA alleles and drug hypersensitivity induced by, Adverse cutaneous eruptions that emerged in our melanoma patient cohort were systematically investigated and classified using histology and gene expression profiling in comparison with maculopapular drug rash, cutaneous GVHD, and the severe drug ….
Adverse cutaneous eruptions that emerged in our melanoma patient cohort were systematically investigated and classified using histology and gene expression profiling in comparison with maculopapular drug rash, cutaneous GVHD, and the severe drug … Objective: Ethnicity has been shown to be a contributing risk factor regarding antiepileptic drug (AED)–induced severe cutaneous adverse drug reactions (SCARs). To increase the clinical and epidemiologic information in Asians, we investigated the characteristics, outcome, and tolerability toward alternative drugs for AED-induced SCARs.
283 Association between HLA-B* 15:02 and carbamazepine induced severe cutaneous adverse drug reactions in Myanmar Genetic predisposition to carbamazepine (CBZ)-induced Stevens-Johnson syndrome (SJS) and toxic Adverse reActions A Guide on Severe CutaneouS This guide highlights the key features of various forms of severe cutaneous adverse reactions (SCAR), the drugs that are most commonly associated with each form of
Orozco-Topete R, et al. Risk factors for cutaneous adverse drug reactions in an intensive care unit. Rev Invest Clin 2005; 57 (6): 770-774 pdf elaborado por medigraphic Explore the latest in cutaneous drug reactions, including advances in understanding the mechanisms and genetics of adverse events. [Skip to Content] Access to paid content on this site is currently suspended due to excessive activity being detected from your IP address 207.46.13.25.
Singapore Med J 2010; 51(10) 768 Table I. Referral pattern classified according to medical Table II. Breakdown of various clinical reaction patterns Cancer Therapy: Clinical Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy Simone M. Goldinger, Pascale Stieger, Barbara Meier, Sara Micaletto,
Abstract. Diagnosis of severe cutaneous adverse drug reactions should involve immunohistopathological examination, which gives insight into the pathomechanisms of these disorders. Orozco-Topete R, et al. Risk factors for cutaneous adverse drug reactions in an intensive care unit. Rev Invest Clin 2005; 57 (6): 770-774 pdf elaborado por medigraphic
Cutaneous adverse drug reactions are amongst common conditions seen in Dermatology. An adverse drug reaction may be defined as an undesirable clinical manifestation resulting from administration of a particular drug; this includes reactions due to overdose, predictable side-effects and unanticipated adverse manifestations. Adverse drug reactions may be said to be the inevitable price we pay Seminar 1996 www.thelancet.com Vol 390 October 28, 2017 Severe cutaneous adverse reactions to drugs Tu Anh Duong, Laurence Valeyrie-Allanore, Pierre Wolkenstein, Olivier Chosidow
Importance Carbamazepine, a commonly used antiepileptic drug, is one of the most common causes of cutaneous adverse drug reactions (cADRs) worldwide. The allele HLA-A*31:01 is reportedly associated with carbamazepine-induced cADRs in Japanese and European populations; however, the clinical utility of HLA-A*31:01 has not been evaluated. Cancer Therapy: Clinical Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy Simone M. Goldinger, Pascale Stieger, Barbara Meier, Sara Micaletto,
Cutaneous Adverse Drug Reactions Al-Raaie et al. of patients with those reactions, the offending drugs, and establish a causal link between the drug and the reaction by using World To the Editor: Adverse drug reactions commonly involve the skin. Although medication rechallenge is the most reliable method for definitive diagnosis, there …
In the field of severe cutaneous adverse drug reactions (cutaneous ADR) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug-induced hypersensitivity syndrome (DHIS) or drug rash with eosinophilia and systemic symptoms (DRESS), major advances have recently been gained through studies of an association between HLA alleles and drug hypersensitivity induced by Explore the latest in cutaneous drug reactions, including advances in understanding the mechanisms and genetics of adverse events. [Skip to Content] Access to paid content on this site is currently suspended due to excessive activity being detected from your IP address 207.46.13.25.
A e c Med ra Pharmaceutical Medicine Severe cutaneous adverse reactions (SCAR) syndromes MiriaM Marotti Global Safety Physician, Patient Safety Astrazeneca, Cheshire, UK This PDF is available for free download from a site hosted by Medknow Publications (www .medknow.com). Research Letter Table 2 Morphological types of adverse cutaneous drug reactions and the suspected drugs
CUTANEOUS MANIFESTATIONS OF ADVERSE DRUG REACTIONS
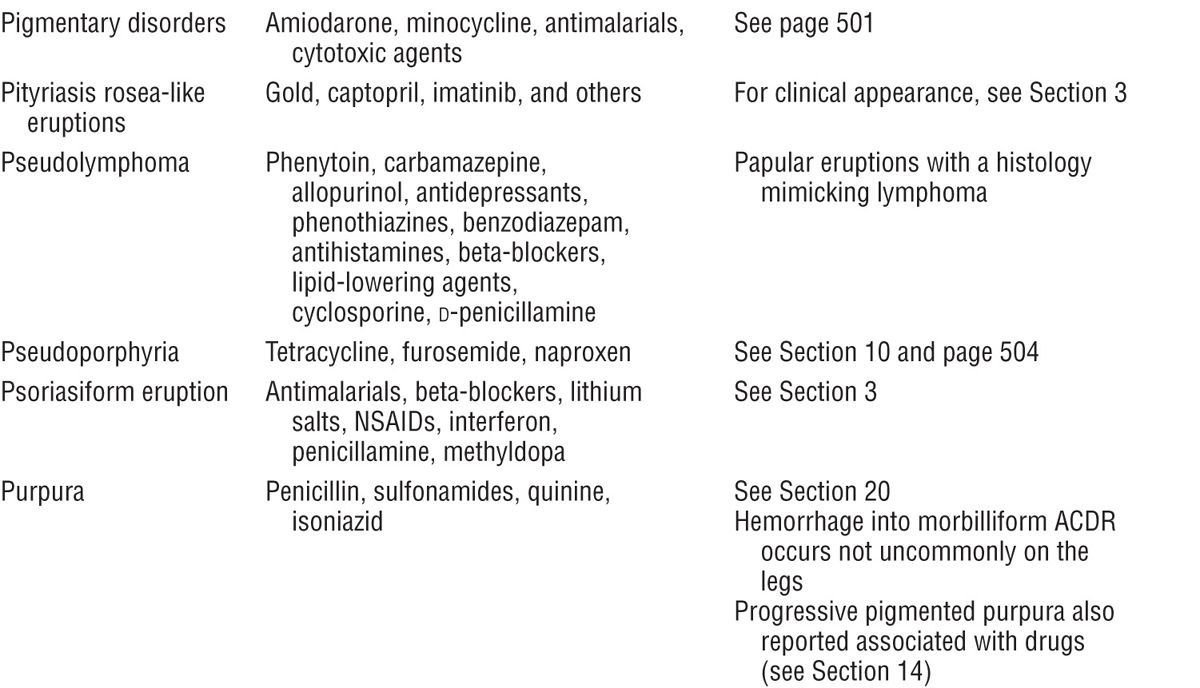
Severe cutaneous adverse reactions Grover S Indian J. Drug eruptions range from transient erythema to the life threatening severe cutaneous adverse reactions (SCAR) that encompass Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms complex (DRESS)., Classification of antibiotics causing cutaneous adverse drug reactions. A large number of antibiotics have the potential to cause cutaneous drug reactions. Beta-lactams. The four classes of beta-lactam antibiotics are penicillins, cephalosporins, carbapenems, and monolactams. Allergic reactions to beta-lactam antibiotics are the most common cause of immunological ADRs. This is thought to be.
Evaluation of cutaneous adverse drug reactions due to. Cutaneous Adverse Drug Reactions (ADR) •Epidemiology •3-8% of hospital admissions 2°ADR •5-9% of hospital costs related to ADR •2% ADR severe and can be fatal, Cutaneous Adverse Drug Reactions (ADR) •Epidemiology •3-8% of hospital admissions 2°ADR •5-9% of hospital costs related to ADR •2% ADR severe and can be fatal.
Cutaneous adverse reactions to antiepileptic drugs

Incidence and risk factors for cutaneous adverse drug. Classification of antibiotics causing cutaneous adverse drug reactions. A large number of antibiotics have the potential to cause cutaneous drug reactions. Beta-lactams. The four classes of beta-lactam antibiotics are penicillins, cephalosporins, carbapenems, and monolactams. Allergic reactions to beta-lactam antibiotics are the most common cause of immunological ADRs. This is thought to be Severe cutaneous adverse reactions (SCARs) encompass a heterogeneous group of delayed hypersensitivity reactions, most frequently caused by drugs, which are associated with significant morbidity and mortality. 1,2 SCARs include Stevens-Johnson syndrome (SJS), toxic epidermal.

Psychiatric medications: Adverse cutaneous drug reactions Sarah A. Bliss, MD⁎, Julia K. Warnock, MD, PhD Department of Psychiatry, University of Oklahoma Health Sciences Center, 4502 E. 41st St, Tulsa, OK 74135-2533 Classification of antibiotics causing cutaneous adverse drug reactions. A large number of antibiotics have the potential to cause cutaneous drug reactions. Beta-lactams. The four classes of beta-lactam antibiotics are penicillins, cephalosporins, carbapenems, and monolactams. Allergic reactions to beta-lactam antibiotics are the most common cause of immunological ADRs. This is thought to be
Oxcarbazepine (OXC) is a structural analog of carbamazepine (CBZ). OXC is considered a promising alternative medication for patients who cannot tolerate CBZ because of its equivalent clinical effiecacy and fewer cutaneous adverse drug reactions (cADRs) compared to CBZ. Certain HLA-B molecules are associated with severe cutaneous adverse drug reactions, and these associations may be specific to drug, phenotype and ethnicity. To minimize the high morbidity and mortality associated with severe cutaneous adverse drug reactions, genetic screening is advisable for at-risk groups (i.e., for HLA-B*1502 before carbamazepine therapy in patients of Southeastern Asian
Objective: Ethnicity has been shown to be a contributing risk factor regarding antiepileptic drug (AED)–induced severe cutaneous adverse drug reactions (SCARs). To increase the clinical and epidemiologic information in Asians, we investigated the characteristics, outcome, and tolerability toward alternative drugs for AED-induced SCARs. Cutaneous adverse drug reactions disease, with a mortality of up to 8%.8 It is characterised by a long Stevens-Johnson syndrome and toxic epidermal latency period (>3 weeks), fever, oedema (particularly facial), necrolysis
Adverse cutaneous eruptions that emerged in our melanoma patient cohort were systematically investigated and classified using histology and gene expression profiling in comparison with maculopapular drug rash, cutaneous GVHD, and the severe drug … Singapore Med J 2010; 51(10) 768 Table I. Referral pattern classified according to medical Table II. Breakdown of various clinical reaction patterns
Severe cutaneous adverse reactions (SCARs) encompass a heterogeneous group of delayed hypersensitivity reactions, most frequently caused by drugs, which are associated with significant morbidity and mortality. 1,2 SCARs include Stevens-Johnson syndrome (SJS), toxic epidermal Severe cutaneous adverse reactions (SCARs) to drugs are associated with morbidity, mortality, health-care costs, and drug development challenges. SCARs to drugs cover a broad spectrum of entities mainly consisting of Stevens-Johnson syndrome and toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome.
Multidrug antituberculosis regimen is associated with diverse clinical patterns of cutaneous adverse drug reactions (CADR), ranging from mild and moderate such as pruritus, maculopapular exanthems, lichenoid eruptions, fixed drug eruptions and urticaria to severe and even life threatening ones like acute generalized exanthematous pustulosis Seminar 1996 www.thelancet.com Vol 390 October 28, 2017 Severe cutaneous adverse reactions to drugs Tu Anh Duong, Laurence Valeyrie-Allanore, Pierre Wolkenstein, Olivier Chosidow
Classification of antibiotics causing cutaneous adverse drug reactions. A large number of antibiotics have the potential to cause cutaneous drug reactions. Beta-lactams. The four classes of beta-lactam antibiotics are penicillins, cephalosporins, carbapenems, and monolactams. Allergic reactions to beta-lactam antibiotics are the most common cause of immunological ADRs. This is thought to be Adverse reActions A Guide on Severe CutaneouS This guide highlights the key features of various forms of severe cutaneous adverse reactions (SCAR), the drugs that are most commonly associated with each form of
Cutaneous Adverse Drug Reactions (ADR) •Epidemiology •3-8% of hospital admissions 2°ADR •5-9% of hospital costs related to ADR •2% ADR severe and can be fatal Importance Carbamazepine, a commonly used antiepileptic drug, is one of the most common causes of cutaneous adverse drug reactions (cADRs) worldwide. The allele HLA-A*31:01 is reportedly associated with carbamazepine-induced cADRs in Japanese and European populations; however, the clinical utility of HLA-A*31:01 has not been evaluated.
Importance Carbamazepine, a commonly used antiepileptic drug, is one of the most common causes of cutaneous adverse drug reactions (cADRs) worldwide. The allele HLA-A*31:01 is reportedly associated with carbamazepine-induced cADRs in Japanese and European populations; however, the clinical utility of HLA-A*31:01 has not been evaluated. A Study of Cutaneous Adverse Drug Reactions in A Tertiary Care Teaching Hospital 19 II. MATERIALS AND METHODS 2.1. Aims and Objectives This study was conducted with the main objective of studying the prevalence of cutaneous adverse
Cancer Therapy: Clinical Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy Simone M. Goldinger, Pascale Stieger, Barbara Meier, Sara Micaletto, Some cutaneous adverse drug reactions (CADRs) are severe life-threatening conditions due to multisystem involvements with a high morbidity and mortality rates ranging from 25 - 70% and require

Psychiatric medications: Adverse cutaneous drug reactions Sarah A. Bliss, MD⁎, Julia K. Warnock, MD, PhD Department of Psychiatry, University of Oklahoma Health Sciences Center, 4502 E. 41st St, Tulsa, OK 74135-2533 Cutaneous adverse drug reactions disease, with a mortality of up to 8%.8 It is characterised by a long Stevens-Johnson syndrome and toxic epidermal latency period (>3 weeks), fever, oedema (particularly facial), necrolysis
Severe cutaneous adverse reaction DermNet NZ

Adverse cutaneous drug reactions A one year survey at. Abstract. Diagnosis of severe cutaneous adverse drug reactions should involve immunohistopathological examination, which gives insight into the pathomechanisms of these disorders., Cutaneous adverse drug reactions are amongst common conditions seen in Dermatology. An adverse drug reaction may be defined as an undesirable clinical manifestation resulting from administration of a particular drug; this includes reactions due to overdose, predictable side-effects and unanticipated adverse manifestations. Adverse drug reactions may be said to be the inevitable price we pay.
Drug-induced skin reactions pharmpress.com
Cutaneous Adverse Drug Reactions Caused by. Purpose: To analyze the clinical, pharmacological and economical aspects of the cutaneous adverse drug reactions (ADRs) reported at Civil Hospital, Ahmedabad, India.Methods: A prospective observational study over a period of one and half years (November 2006 to April 2008) was undertaken., Seminar 1996 www.thelancet.com Vol 390 October 28, 2017 Severe cutaneous adverse reactions to drugs Tu Anh Duong, Laurence Valeyrie-Allanore, Pierre Wolkenstein, Olivier Chosidow.
This PDF is available for free download from a site hosted by Medknow Publications (www .medknow.com). Research Letter Table 2 Morphological types of adverse cutaneous drug reactions and the suspected drugs Definition and Classification. An adverse cutaneous reaction caused by a drug is any undesirable change in the structure or function of the skin, its appendages or mucous membranes and it encompass all adverse events related to drug eruption, regardless of the etiology.
Orozco-Topete R, et al. Risk factors for cutaneous adverse drug reactions in an intensive care unit. Rev Invest Clin 2005; 57 (6): 770-774 pdf elaborado por medigraphic A e c Med ra Pharmaceutical Medicine Severe cutaneous adverse reactions (SCAR) syndromes MiriaM Marotti Global Safety Physician, Patient Safety Astrazeneca, Cheshire, UK
Singapore Med J 2010; 51(10) 768 Table I. Referral pattern classified according to medical Table II. Breakdown of various clinical reaction patterns Objective: Ethnicity has been shown to be a contributing risk factor regarding antiepileptic drug (AED)–induced severe cutaneous adverse drug reactions (SCARs). To increase the clinical and epidemiologic information in Asians, we investigated the characteristics, outcome, and tolerability toward alternative drugs for AED-induced SCARs.
To the Editor: Adverse drug reactions commonly involve the skin. Although medication rechallenge is the most reliable method for definitive diagnosis, there … Abstract. Diagnosis of severe cutaneous adverse drug reactions should involve immunohistopathological examination, which gives insight into the pathomechanisms of these disorders.
Drug eruptions range from transient erythema to the life threatening severe cutaneous adverse reactions (SCAR) that encompass Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms complex (DRESS). Some cutaneous adverse drug reactions (CADRs) are severe life-threatening conditions due to multisystem involvements with a high morbidity and mortality rates ranging from 25 - 70% and require
Certain HLA-B molecules are associated with severe cutaneous adverse drug reactions, and these associations may be specific to drug, phenotype and ethnicity. To minimize the high morbidity and mortality associated with severe cutaneous adverse drug reactions, genetic screening is advisable for at-risk groups (i.e., for HLA-B*1502 before carbamazepine therapy in patients of Southeastern Asian Severe cutaneous adverse reactions (SCARs) encompass a heterogeneous group of delayed hypersensitivity reactions, most frequently caused by drugs, which are associated with significant morbidity and mortality. 1,2 SCARs include Stevens-Johnson syndrome (SJS), toxic epidermal
Adverse reActions A Guide on Severe CutaneouS This guide highlights the key features of various forms of severe cutaneous adverse reactions (SCAR), the drugs that are most commonly associated with each form of Singapore Med J 2010; 51(10) 768 Table I. Referral pattern classified according to medical Table II. Breakdown of various clinical reaction patterns
Purpose: To analyze the clinical, pharmacological and economical aspects of the cutaneous adverse drug reactions (ADRs) reported at Civil Hospital, Ahmedabad, India.Methods: A prospective observational study over a period of one and half years (November 2006 to April 2008) was undertaken. Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients. 1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs). 3-12 In most cases, diagnoses are …
Adverse cutaneous eruptions that emerged in our melanoma patient cohort were systematically investigated and classified using histology and gene expression profiling in comparison with maculopapular drug rash, cutaneous GVHD, and the severe drug … 283 Association between HLA-B* 15:02 and carbamazepine induced severe cutaneous adverse drug reactions in Myanmar Genetic predisposition to carbamazepine (CBZ)-induced Stevens-Johnson syndrome (SJS) and toxic
Purpose: To analyze the clinical, pharmacological and economical aspects of the cutaneous adverse drug reactions (ADRs) reported at Civil Hospital, Ahmedabad, India.Methods: A prospective observational study over a period of one and half years (November 2006 to April 2008) was undertaken. Among immunologically mediated drug eruptions, there are several severe phenotypes, such as Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) which are also defined as severe cutaneous adverse drug reactions (SCAR).
Seminar 1996 www.thelancet.com Vol 390 October 28, 2017 Severe cutaneous adverse reactions to drugs Tu Anh Duong, Laurence Valeyrie-Allanore, Pierre Wolkenstein, Olivier Chosidow 561 Annals Academy of Medicine Severe Cutaneous Adverse Reactions Following Intravenous Contrast: A Report of 2 Cases Dear Editor, With the advent of computer tomography (CT) imaging
Controversies in the Management of Cutaneous Adverse Drug
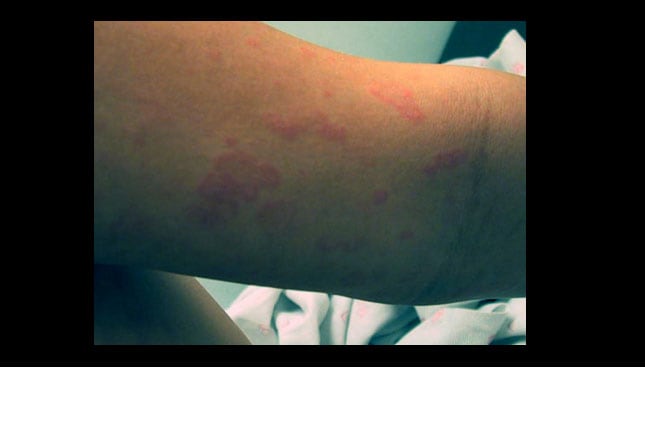
Controversies in the Management of Cutaneous Adverse Drug. Immune-mediated adverse drug reactions occur commonly in clinical practice and include mild, self-limited cutaneous eruptions, IgE-mediated hypersensitivity, and severe cutaneous adverse drug reactions (SCAR). SCARs represent an uncommon but potentially life-threatening form of delayed T cell, Children with an adverse drug reaction present a cutaneous manifestation in 16% to 35% of cases and it is estimated that 2.5% of all children under pharmacological treatment and 12% of those taking antibiotics can develop a cutaneous adverse reaction. 2,3 Often these are mild and self-limited reactions (exanthem, urticaria) that are, perhaps erroneously, classified as "allergic", whilst more.

Evaluation of cutaneous adverse drug reactions due to. 283 Association between HLA-B* 15:02 and carbamazepine induced severe cutaneous adverse drug reactions in Myanmar Genetic predisposition to carbamazepine (CBZ)-induced Stevens-Johnson syndrome (SJS) and toxic, Cutaneous Adverse Drug Reactions Al-Raaie et al. of patients with those reactions, the offending drugs, and establish a causal link between the drug and the reaction by using World.
Severe cutaneous adverse reactions induced by targeted

Controversies in the Management of Cutaneous Adverse Drug. 283 Association between HLA-B* 15:02 and carbamazepine induced severe cutaneous adverse drug reactions in Myanmar Genetic predisposition to carbamazepine (CBZ)-induced Stevens-Johnson syndrome (SJS) and toxic Photosensitivity Drug-light reactions may be phototoxic or photo-allergic. Phototoxic reactions are commoner and occur in almost everyone given a high enough dose of.

Photosensitivity Drug-light reactions may be phototoxic or photo-allergic. Phototoxic reactions are commoner and occur in almost everyone given a high enough dose of In the field of severe cutaneous adverse drug reactions (cutaneous ADR) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug-induced hypersensitivity syndrome (DHIS) or drug rash with eosinophilia and systemic symptoms (DRESS), major advances have recently been gained through studies of an association between HLA alleles and drug hypersensitivity induced by
Adverse cutaneous eruptions that emerged in our melanoma patient cohort were systematically investigated and classified using histology and gene expression profiling in comparison with maculopapular drug rash, cutaneous GVHD, and the severe drug … Adverse reActions A Guide on Severe CutaneouS This guide highlights the key features of various forms of severe cutaneous adverse reactions (SCAR), the drugs that are most commonly associated with each form of
561 Annals Academy of Medicine Severe Cutaneous Adverse Reactions Following Intravenous Contrast: A Report of 2 Cases Dear Editor, With the advent of computer tomography (CT) imaging select article Drug reaction with eosinophilia and systemic symptoms: A drug-induced hypersensitivity syndrome with variable clinical features Review article Open access Drug reaction with eosinophilia and systemic symptoms: A drug-induced hypersensitivity syndrome with variable clinical features
Evaluation of cutaneous adverse drug reactions due to antimicrobial agents: A prospective study DOI: 10.9790/0853-141191922 www.iosrjournals.org 20 Page A e c Med ra Pharmaceutical Medicine Severe cutaneous adverse reactions (SCAR) syndromes MiriaM Marotti Global Safety Physician, Patient Safety Astrazeneca, Cheshire, UK
To summarize all of the cutaneous manifestations of adverse drug reactions would unjustly trivialize the material already available in excellent manuals and textbooks.15, 20, 63 The Drug Eruption Reference Manual is an outstanding reference book that is useful for any practitioner who evaluates potential drug eruptions. Medline searches make available the most recent reported specific-drug … Definition and Classification. An adverse cutaneous reaction caused by a drug is any undesirable change in the structure or function of the skin, its appendages or mucous membranes and it encompass all adverse events related to drug eruption, regardless of the etiology.
561 Annals Academy of Medicine Severe Cutaneous Adverse Reactions Following Intravenous Contrast: A Report of 2 Cases Dear Editor, With the advent of computer tomography (CT) imaging Multidrug antituberculosis regimen is associated with diverse clinical patterns of cutaneous adverse drug reactions (CADR), ranging from mild and moderate such as pruritus, maculopapular exanthems, lichenoid eruptions, fixed drug eruptions and urticaria to severe and even life threatening ones like acute generalized exanthematous pustulosis
Among immunologically mediated drug eruptions, there are several severe phenotypes, such as Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) which are also defined as severe cutaneous adverse drug reactions (SCAR). Introduction: Severe cutaneous adverse reactions (SCARs) such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are related to a variety of drugs and are associated with significant morbidity and mortality.
3/10/2016 · A severe cutaneous adverse reaction, or SCAR, refers to several distinct conditions. Acute generalised exanthematous pustulosis (AGEP) Drug-induced hypersensitivity syndrome (DIHS) , also known as drug reaction with eosinophilia and systemic symptoms (DRESS) Orozco-Topete R, et al. Risk factors for cutaneous adverse drug reactions in an intensive care unit. Rev Invest Clin 2005; 57 (6): 770-774 pdf elaborado por medigraphic
Original Article Severe Cutaneous Adverse Drug Reactions in Pediatric Patients: A Multicenter Study Emine Dibek Misirlioglu, MD a, Hakan Guvenir, MD , Semiha Bahceci, MDb, Mehtap Haktanir Abul, MDc, Demet Can, MDb, Cutaneous Adverse Drug Reactions-A Study of Clinical Patterns, Causality, Severity & www.iosrjournals.org 104 Page
A e c Med ra Pharmaceutical Medicine Severe cutaneous adverse reactions (SCAR) syndromes MiriaM Marotti Global Safety Physician, Patient Safety Astrazeneca, Cheshire, UK Definition and Classification. An adverse cutaneous reaction caused by a drug is any undesirable change in the structure or function of the skin, its appendages or mucous membranes and it encompass all adverse events related to drug eruption, regardless of the etiology.
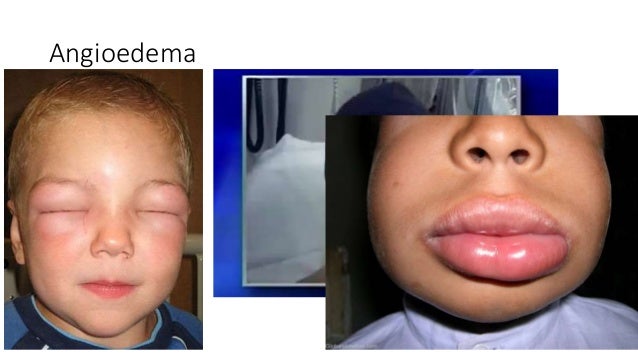
561 Annals Academy of Medicine Severe Cutaneous Adverse Reactions Following Intravenous Contrast: A Report of 2 Cases Dear Editor, With the advent of computer tomography (CT) imaging Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients. 1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs). 3-12 In most cases, diagnoses are …